Welcome to Morbus & Curis, a blog about disease and healthcare. Today’s blog post takes a look at how some of the procedures concerning marketing authorisations and variations will change in the UK from the 1st January 2020.
Brexit is almost upon us and when the clocks around the UK strike 11 pm on the 31st December many changes will happen to the way medicines are granted marketing authorisations and variations in Great Britain.
The MHRA has issued some updated guidance on its website and more guidance for Northern Ireland will follow this month.
Let’s take a look at some of the changes in store for the pharmaceutical and biotechnology industries.
Nomenclature
Before diving into the technical details, here’s a brief overview of some of the terms and acronyms that will be used throughout this blog post:
Note: Centrally Authorised Products (CAPs) are those that have been granted Marketing Authorisation by the EMA after being assessed on a Community level involving all EU Member States.
Grandfathering of CAPs
On 1st January 2021, European MAs will be converted into national MAs (a process called grandfathering). While this is a necessary step for all medicines marketed in England, Scotland and Wales, in Northern Ireland CAP MAs will remain valid as Northern Ireland continues to be within in the scope of the centralised authorisation procedure.
Great Britain
CAP MAs will be automatically converted into UK MAs (which will also be known as “converted EU MAs”) that are only effective and valid in Great Britain only.
Although the process is automatic some administrative work will be required. The MAH will need to submit important data and information about their medicine to the MHRA via an initiating electronic Common Technical Document (eCTD). This data must be submitted by 1st January 2022.
While there is no conversion fee, UK MAHs will subsequently be required to pay an annual service fee to the MHRA.
UK MAs will have a PLGB XXXXX/YYYY format (XXXXX being the company number) and a single MA number will cover all pack sizes of a presentation. If the MHRA has not allocated a MAH a company number they must complete a company number application form; one will not be generated automatically.
MAs for CAPs that are not currently marketed in the EU or UK can also be converted.
MAHs can opt-out of the conversion process by notifying the MHRA but will subsequently no longer be permitted to market their medicine in Great Britain. The opt-out deadline is 21 January 2021.
Lastly, the MHRA will subsequently publish a list of products that have and have not been converted.
Northern Ireland
In Northern Ireland, CAP MAs will remain valid and subject to EU regulations as the country will remain in the scope of the Centralised Procedure.
Read more here.
New Assessment Routes
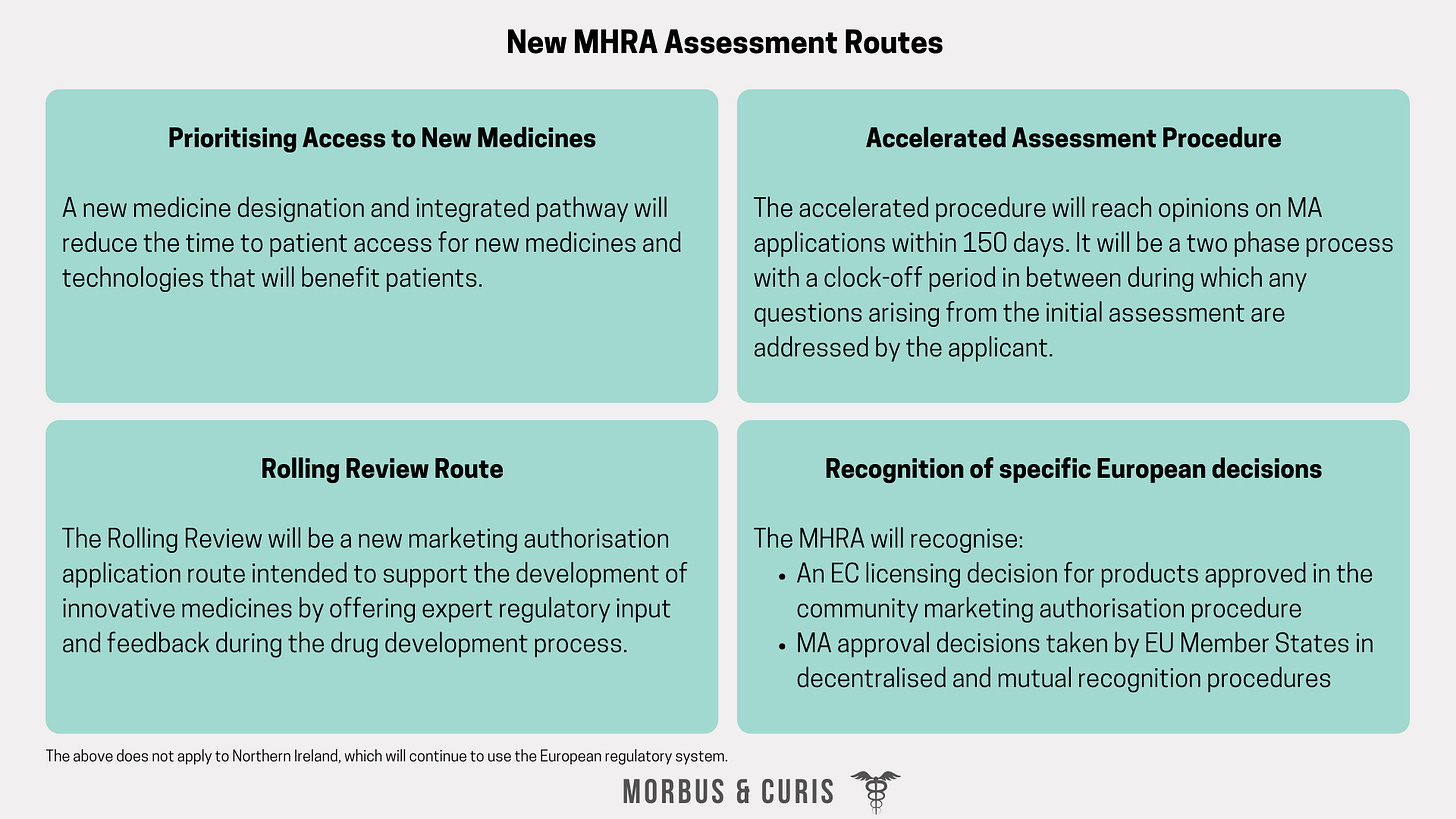
National licensing procedures conducted by the MHRA will change from 1st January 2021, including the establishment of new evaluation routes for innovative medicines.
Changes include:
Procedures to prioritise access to new medicines
An accelerated assessment procedure
A rolling review route for MA applications
Recognition of a European Commission licensing decision for products approved in the community marketing authorisation procedure and recognition of marketing authorisation approval decisions taken by European Union Member States in decentralised and mutual recognition procedures.
These new assessment routes will only apply in Great Britain. Northern Ireland will continue to use the European regulatory system.
Prioritising Access to New Medicines
The MHRA is developing new approaches that will reduce the time to patient access for new medicines and technologies with demonstrated patient benefit.
It will include a new medicine designation and an integrated pathway to patient access in Great Britain.
The pathway will include a development support toolkit and a platform for sustained multi-stakeholder interactions to support and advise on evidence requirements.
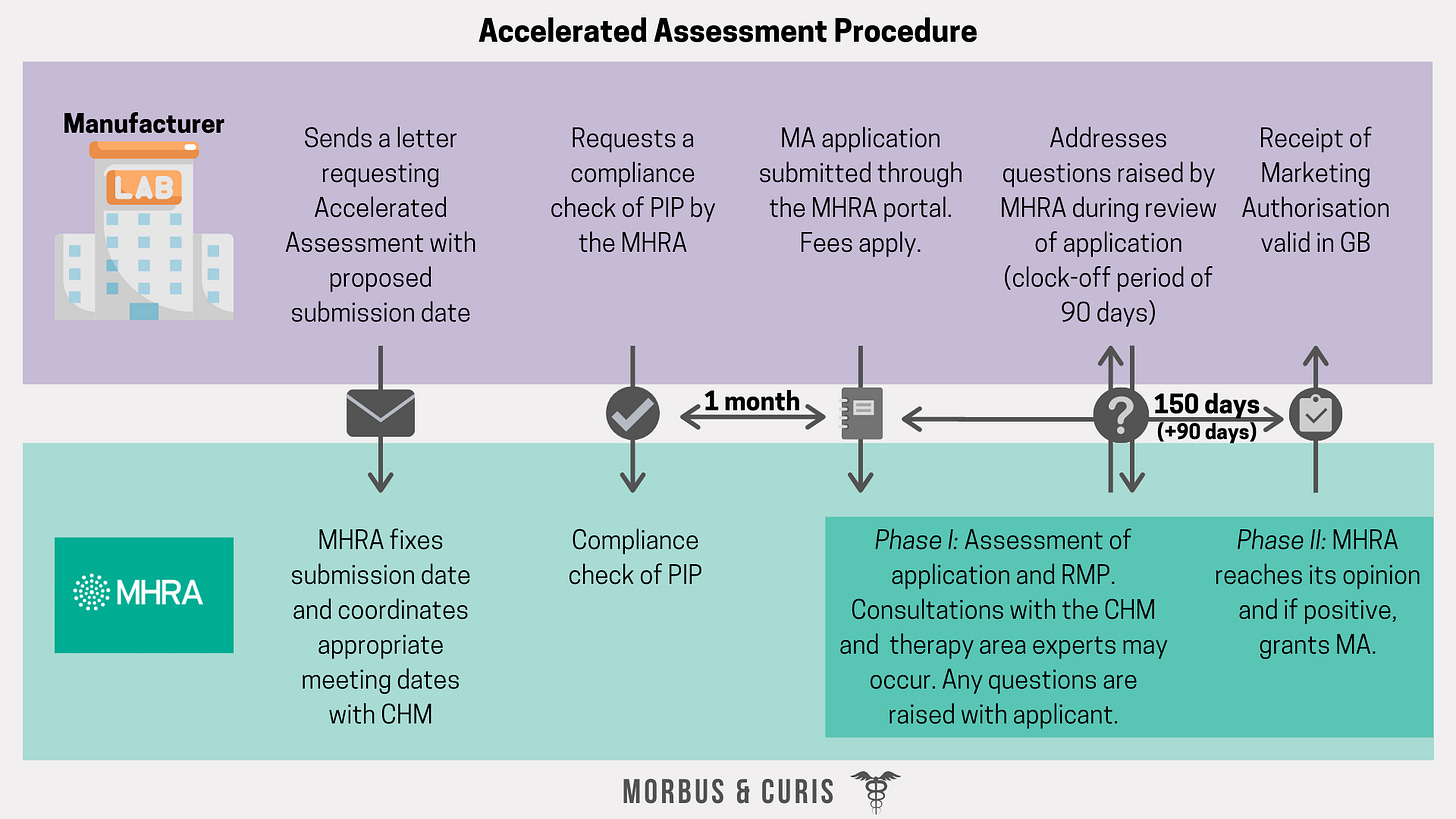
Accelerated Assessment Procedure
An accelerated procedure will be introduced by the MHRA. The procedure will require opinions on MA applications to be reached within 150 days. It will be a two-phase process with the second phase commencing after a clock-off period during which any questions arising from the initial assessment are addressed by the applicant.
Steps:
Prior to a submission: the MHRA should be notified in advance via a letter detailing the expected submission date, and a compliance check of the PIP should be conducted.
Submission: Application submitted through the MHRA portal. Applications should include a UK specific CTD module 1, CTDs 2-5 and a Risk Management Plan. They must also be compliant with GB paediatric requirements and investigation plans.
Phase I: The Licensing Division of MHRA assesses application while the Vigilance and Risk Management of Medicines Division evaluates the Risk Management Plan. If necessary, consultations with the CHM and input from therapy area experts may occur.
Clock-off period: questions arising from phase I assessment are raised with then addressed by the applicant.
Phase II: MHRA reaches its opinion and, if positive, grants MA. MHRA also determines eligibility for orphan status or a conditional MA.
Publication: once the assessment has concluded it will be published via a Public Assessment Report for the product.
The accelerated assessment will be available for new MA applications for new and existing active substances, which may be conditional, full or orphan MA applications. Submissions for approval under exceptional circumstances may also use the procedure.
Rolling Review Route
The Rolling Review will be a new marketing authorisation application route established by the MHRA.
It’s intended to support the development of innovative medicines by offering expert regulatory input and feedback during the drug development process. It’s hoped that this early, iterative engagement will allow for efficient assessments and approvals of final, complete MA applications.
Some details are yet to be published, but here are some of the Rolling Review details revealed thus far:
The rolling review route will be available for applications for any new active ingredient (both small molecular weight and biological products, including biosimilars)
Modules for review may be submitted together or separately. This will permit the manufacturer greater flexibility with regards to data availability.
Module assessments will progress independently, in consultation with expert advisory groups.
After receiving feedback on each module from the MHRA, applicants can update them prior to their final MA application submission, which must also contain an RMP and be compliant with GB paediatric requirements.
Final MA decisions will be made in consultation with the CHM, therapy area experts and other expert advisory groups as necessary
Recognition of specific European decisions
Certain licensing and Marketing Authorisation decisions made by the European Commission will be recognised and adopted by Great Britain from 1st January 2021 to 1st January 2023.
This will not happen automatically; applications must be made to the MHRA and they will need to:
Include all information provided to the EMA during the licensing procedure
Include all iterations of the CHMP assessment report and final opinion
Include a declaration of conformity between the GB application and EMA approved dossier
Comply with all UK specific requirements
Additionally, when assessing UK MA applications, the MHRA will consider MA decisions made by EU member states via decentralised or mutual recognition procedures, if UK applications:
Include all information submitted to the reference Member State
Include all iterations of the RMS assessment report, including the RMS end of procedure notification
Conform with the dossier approved in the RMS
The UK will also have the power to take into account marketing authorisation decisions of EU Member States when considering applications for marketing authorisations for products that have been approved in decentralised or mutual recognition procedures.
Applications should include all information submitted to the reference Member State and accompanied by all iterations of the RMS assessment report, including the RMS end of procedure notification. A declaration of conformity of the UK application with the dossier approved in the RMS should be provided.
The application should be submitted to MHRA following receipt of the RMS end of procedure notification.
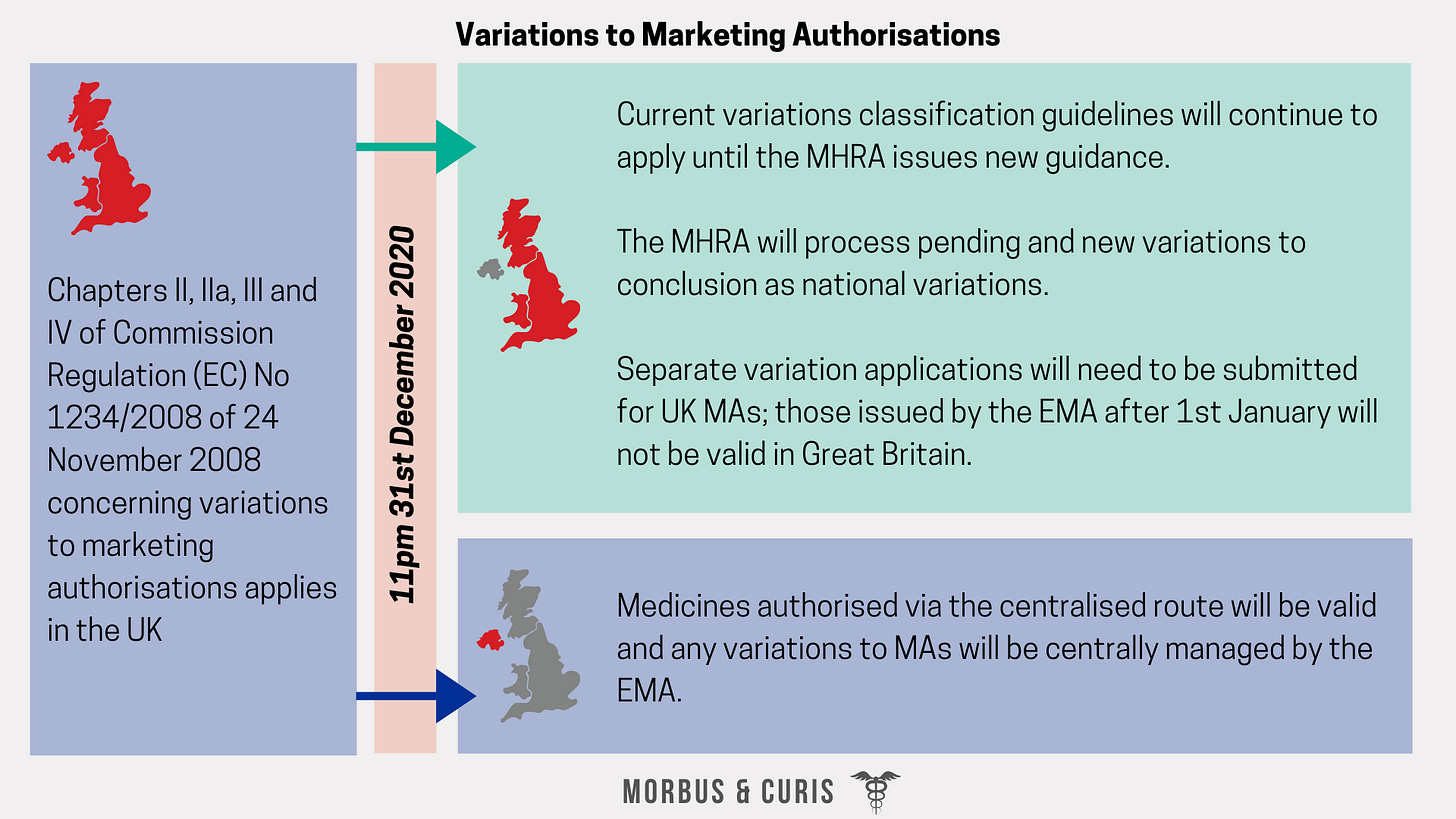
Variations to Marketing Authorisations
An overview of the different types of Marketing Authorisation variations (Type IA, Type IAIN, Type IB, Type II or Extension) that are currently used in Europe can be found here.
Great Britain
Guidance issued thus far for Great Britain includes the following:
Variation Classification Guidelines: Classification guidelines, as well as conditions that must be met to qualify for a variation and supporting documentation required for a variation application, will continue to apply in the UK until the MHRA issues revised guidance. Any specific requests concerning the classification of a variation that are still pending on 1st January 2021 will need to be submitted directly to the MHRA, who will issue its own recommendation.
Variation Procedures: existing regulations for variations to national Marketing Authorisations, will be incorporated into UK law.
Pending Variations:
Pending variations to national MAs: will be processed to conclusion using existing national procedures.
Pending variations covered by mutual recognition/decentralised procedures or that are part of a worksharing procedure: where a decision has already been made on a variation procedure conducted by a Reference Member State (that is not the UK) but the final decision has not been processed in the UK by 1st January 2021, the MHRA will implement the agreed outcome of the variation procedure.
New Variation Applications: New variations submitted from 1 January 2021 will be processed as national variations, using the same transposed procedures.
Northern Ireland
Under the Northern Ireland Protocol, medicines authorised via the centralised route will be valid in Northern Ireland and any variations to these Marketing Authorisations will be centrally managed by the EMA.
For further information about variations and guidance for changing the finished product manufacturer, Marketing Authorisation Holder, importer, batch release site or quality control site etc., read more here.
Wrap up
While in Great Britain new marketing authorisations will be issued for medicines that continue to be marketed there and new MHRA assessment routes will come into effect, Northern Ireland will largely be treated like it is part of the EEA with decisions made by the EMA/EC being relevant and valid there, notably marketing authorisation decisions and CE marks.
For further MHRA Brexit guidance on other topics such as clinical trials, pharmacovigilance procedures and new IT systems see the MHRA website.
Sources
If you found this blog post helpful why not consider subscribing or sharing this post.









what is the "annual service fee". Didn't find it on the MHRA link
https://www.gov.uk/government/publications/mhra-fees