National Medicine Reimbursement and Pricing Decision Making in France
Welcome to Morbus & Curis, a blog about disease and healthcare. Today’s blog post is an overview of the national decision-making process for medicinal product reimbursement and pricing in France.

Key Decision Making Bodies involved in Reimbursement Decision Making
Multiple authorities have a role in medicine reimbursement and pricing decision making in France. These include:

We’ll dig deeper into the role of each body throughout the article, but in short:
The ANSM is the French national regulatory authority. It is authorised to monitor and regulate pharmaceuticals, biological products and medical devices.
HAS is the French National Authority for Health. Its activities are diverse and include the assessment of medicines, the publication of guidelines, the accreditation of healthcare organisations and the certification of doctors.
The CT assesses medicinal products with Marketing Authorisation.
The CEESP conducts health economic assessments of certain medicines.
CEPS negotiates pricing with manufacturers.
UNCAM decides the reimbursement rate of products recommended for inclusion on the positive reimbursement list.
Regulatory Approval and Marketing Authorisation
Before a reimbursement application can be made, a medicine must first be granted Marketing Authorisation from either the European Medicines Agency (EMA) via a centralised procedure or the ANSM via the national procedure.
The EMA’s centralised procedure is compulsory for certain drugs such as those derived from biotechnology processes, advanced therapy drugs and orphan drugs. Additionally, drugs indicated for the treatment of certain diseases including HIV, cancer and diabetes, as well as autoimmune, neurodegenerative and viral diseases.
The centralised procedure has a time limit of 210 days but this period is reduced for medicinal products of major public health interest, which are eligible to be reviewed under an accelerated assessment procedure.

The Reimbursement Decision Making Process
Once a medicinal product has Marketing Authorisation its manufacturer can apply to HAS to have the product assessed for national reimbursement.
This application may be for an initial reimbursement listing, a listing renewable or the inclusion of a new or extended indication for an already reimbursed product.
There are two lists of reimbursable medicines in France; one for medicines dispensed in hospital pharmacies and one for those dispensed by retail pharmacies. A manufacturer may apply to have their product included in one or both.
Reimbursement applications require a technical dossier, which is submitted to the CT, and an economic dossier, which may be evaluated by the CEESP, as well as CEPS.
The process begins with a technical assessment conducted by the CT and, in parallel, the CEESP may independently conduct a health economic assessment of the medical product.
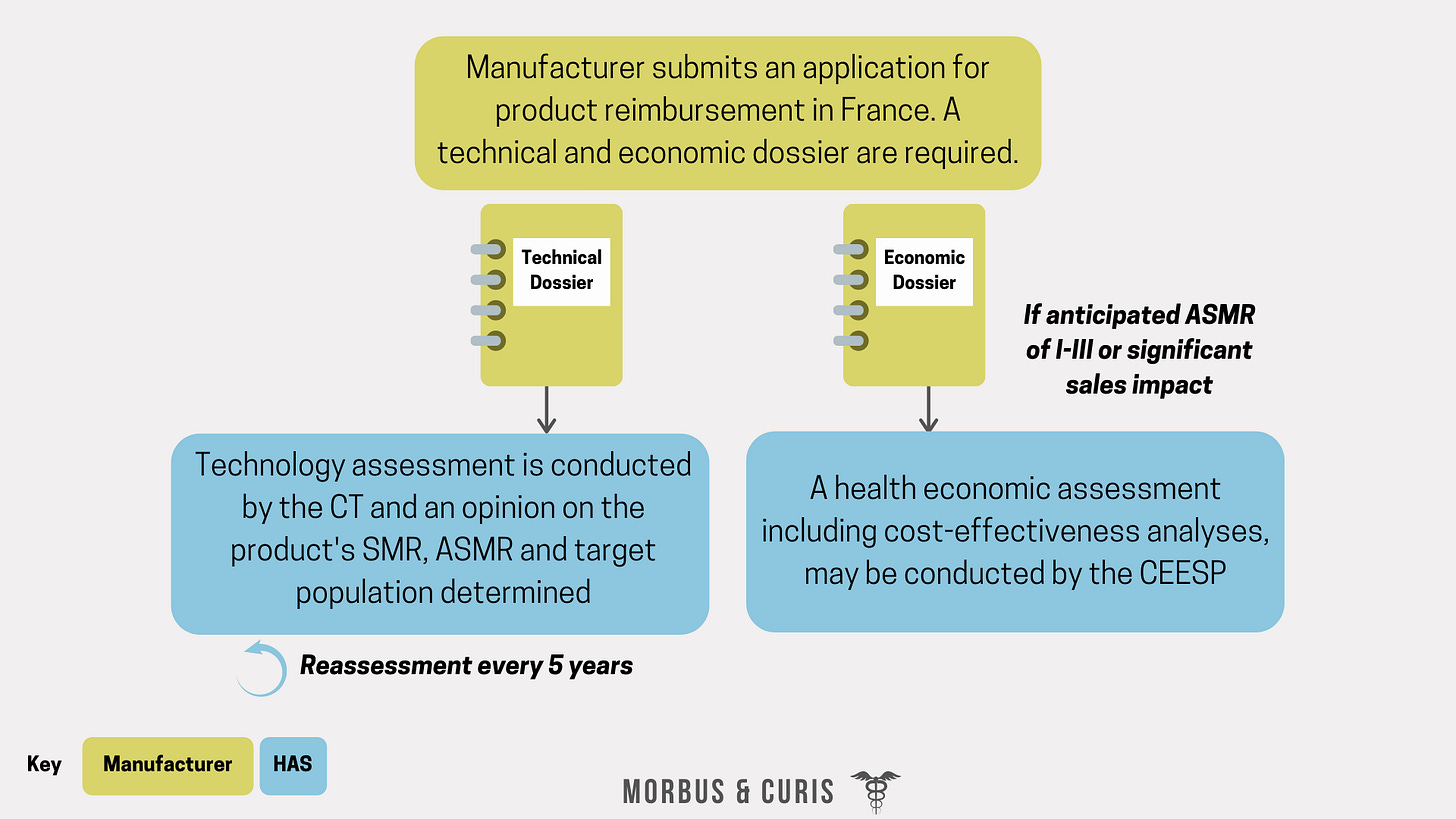
There are three key outcomes from the CT’s technical assessment; the treatment’s actual medical benefit (SMR), the improvement in the actual medical benefit (ASMR) and the target patient population eligible for treatment.

The CEESP may assess the cost-effectiveness of a product if the following criteria are met:
The manufacturer submits for an ASMR of I-III.
The product is anticipated to have a significant impact on the health insurance budget (defined as more than €20 million in annual revenue).
The methodology for CEESP’s health economic evaluation is defined by HAS and the assessment is not shared with the CT until it is final.
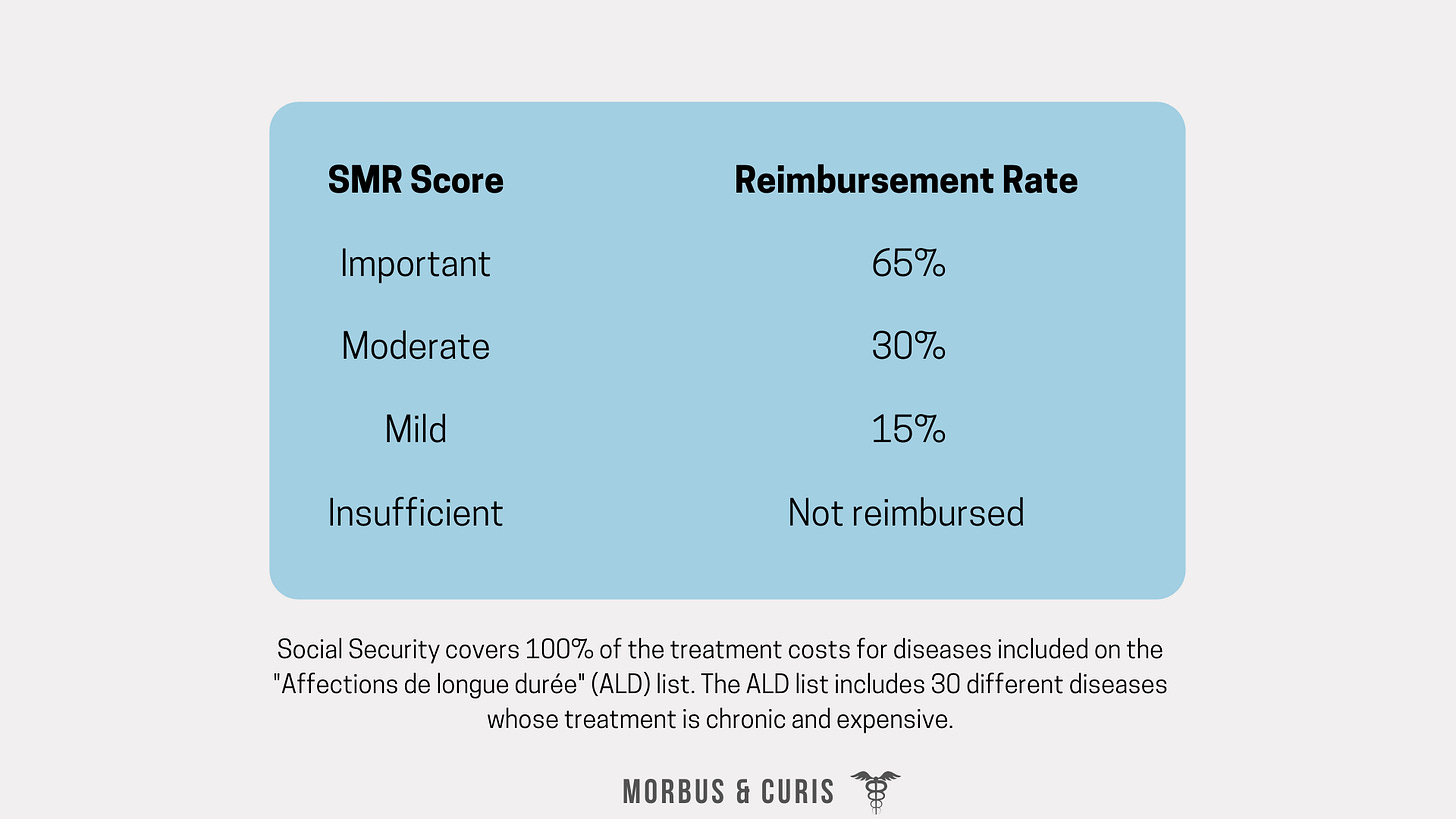
Subsequently, the CT’s SMR score informs the reimbursement rate that should be granted to a treatment. This is primarily decided by UNCAM, although final approval sits with HAS.
The CT’s ASMR score informs the pricing potential of a treatment, which is negotiated between the manufacturer and CEPS. Once a price is agreed it is published in the Official Gazette.

The SMR score typically informs reimbursement rates as follows:
The French reimbursement system has two main parts; compulsory and supplementary.
The compulsory health insurance scheme is, as the name suggests, compulsory and contributions are income-based.
Supplementary health insurance schemes are variable and cover some of the health care expenses that are not covered by the compulsory scheme.
In most cases, people with supplementary health insurance often have the full cost of a treatment reimbursed as their supplementary insurance covers the remaining cost of a treatment that is not fully covered by the compulsory scheme.
The ASMR score typically informs pricing potential as follows:

In addition to negotiating the public price, CEPS can negotiate price/volume agreements with the manufacturer. These agreements are negotiated considering key factors such as:
The target patient population size
Estimated real-world usage
Initial price point submitted by the manufacturer
The pricing of relevant comparators
3-year sales forecast for the product
CEPS does not negotiate pricing for:
Hospital drugs included in the Tarification à l’Activité (T2A ). Prices for these drugs are unregulated and their costs are covered by a Diagnosis Related Group (DRG) payment model.
Expensive hospital drugs that are not included in the T2A. Prices for these drugs are set and registered by the manufacturer. These drugs are fully reimbursed by the health insurance system.
Prices are unregulated for medicines that are not covered by the French reimbursement system.
Once the reimbursement rate and price decisions are finalised and published, the inclusion of the medicine on the reimbursement list is valid for 5 years. After 5 years, an updated dossier needs to be submitted to the CT who then reassesses the medicine’s SMR and ASMR.
If significant new information or evidence becomes available earlier, then a reassessment by the CT can occur sooner.
Summary of the Process

Sources
If you found this blog post helpful why not consider subscribing.
Cover photo credit: Chris Karidis on Unsplash




