What are Rare Diseases?
Welcome to Morbus & Curis, a blog about disease and healthcare. Today’s blog post is a brief overview of rare diseases.
What is a rare disease?
The precise definition of a rare disease varies around the world.
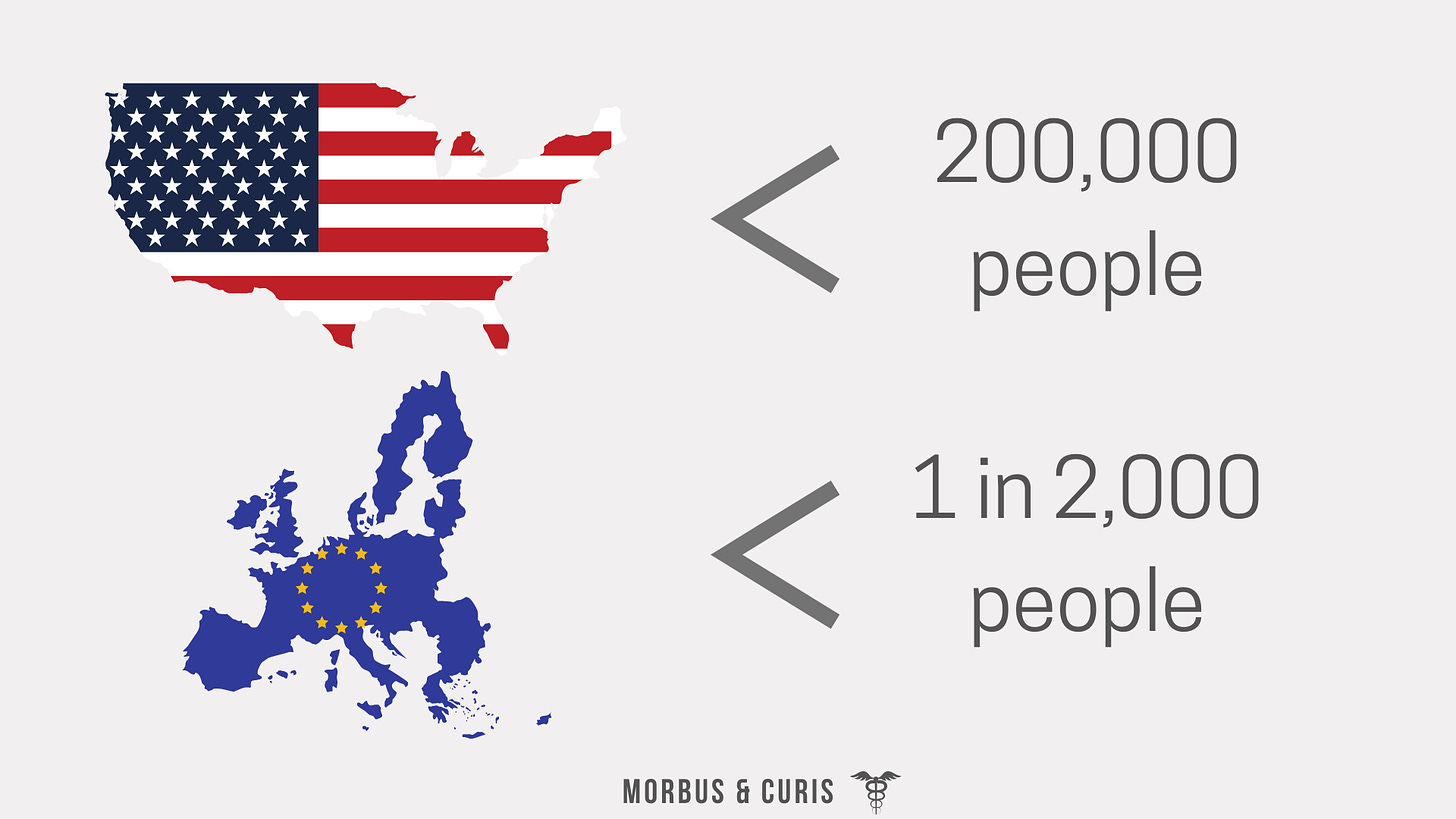
In the United States, it’s defined as a disease affecting fewer than 200,000 people in the US.
In Europe, it’s defined as a disease affecting fewer than 1 in 2,000 people (commonly expressed as 5 in 10,000).
This quantifiable definition is helpful for regulators since it allows them to objectively determine which medicines should be designated as orphan medicines (medicines treating rare diseases) and qualify for certain R&D incentive programmes.
How many rare diseases are there?
It’s estimated that there are somewhere between 6,000-8,000 different rare diseases.
The prevalence of different rare diseases varies hugely - just 350 rare diseases account for 80% of people living with a rare disease.
How many people are living with a rare disease?
While the prevalence of an individual rare disease may be low, collectively they affect a significant proportion of the general population.
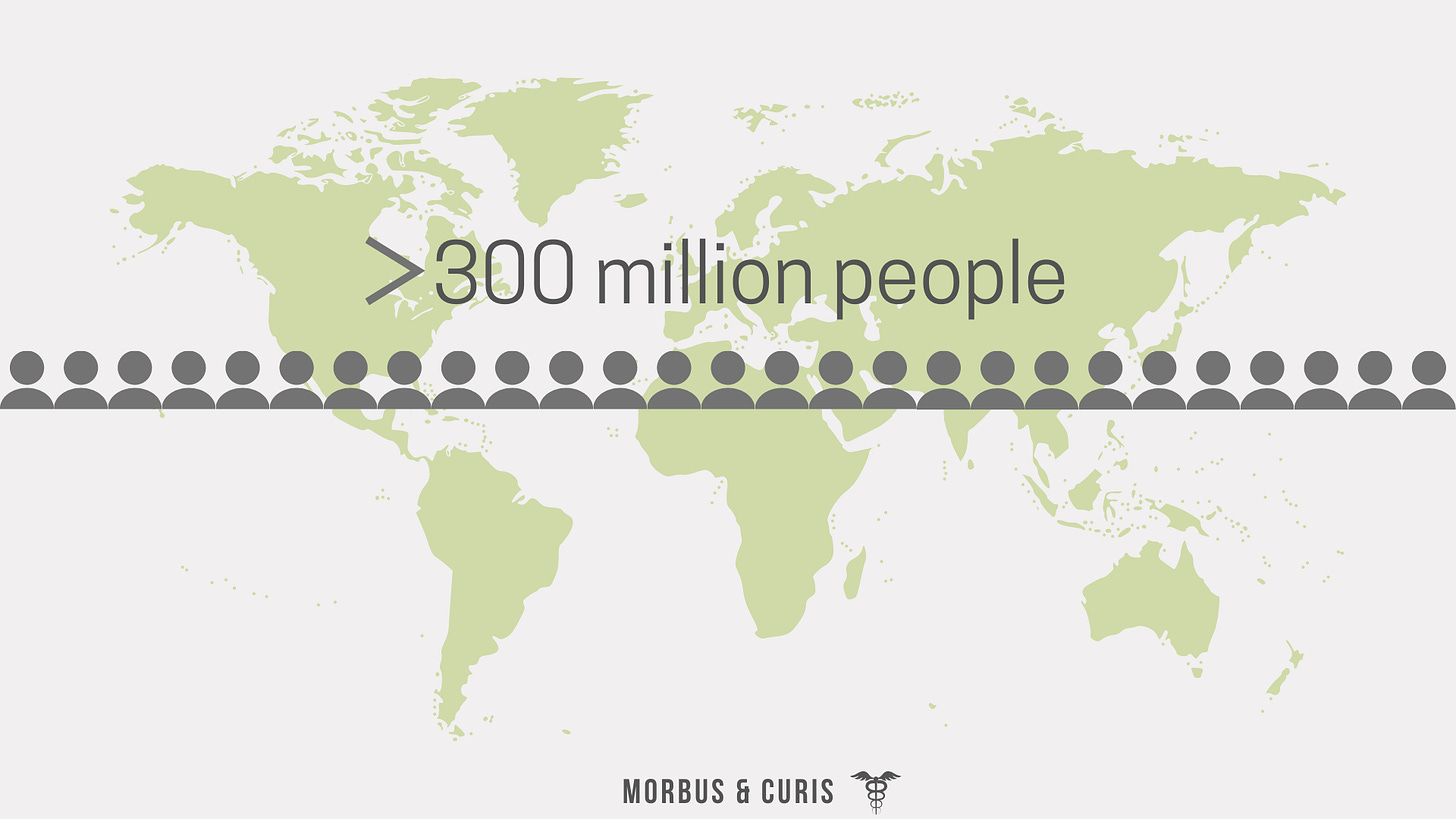
Globally it’s estimated that 300-400 million people are living with a rare disease (that’s almost 1 in every 15 people and more than the population of the US - the third most populous country in the world).
In the US, it’s estimated that the total number of people living with a rare disease is 25 million, and in Europe, the estimate is 30 million.
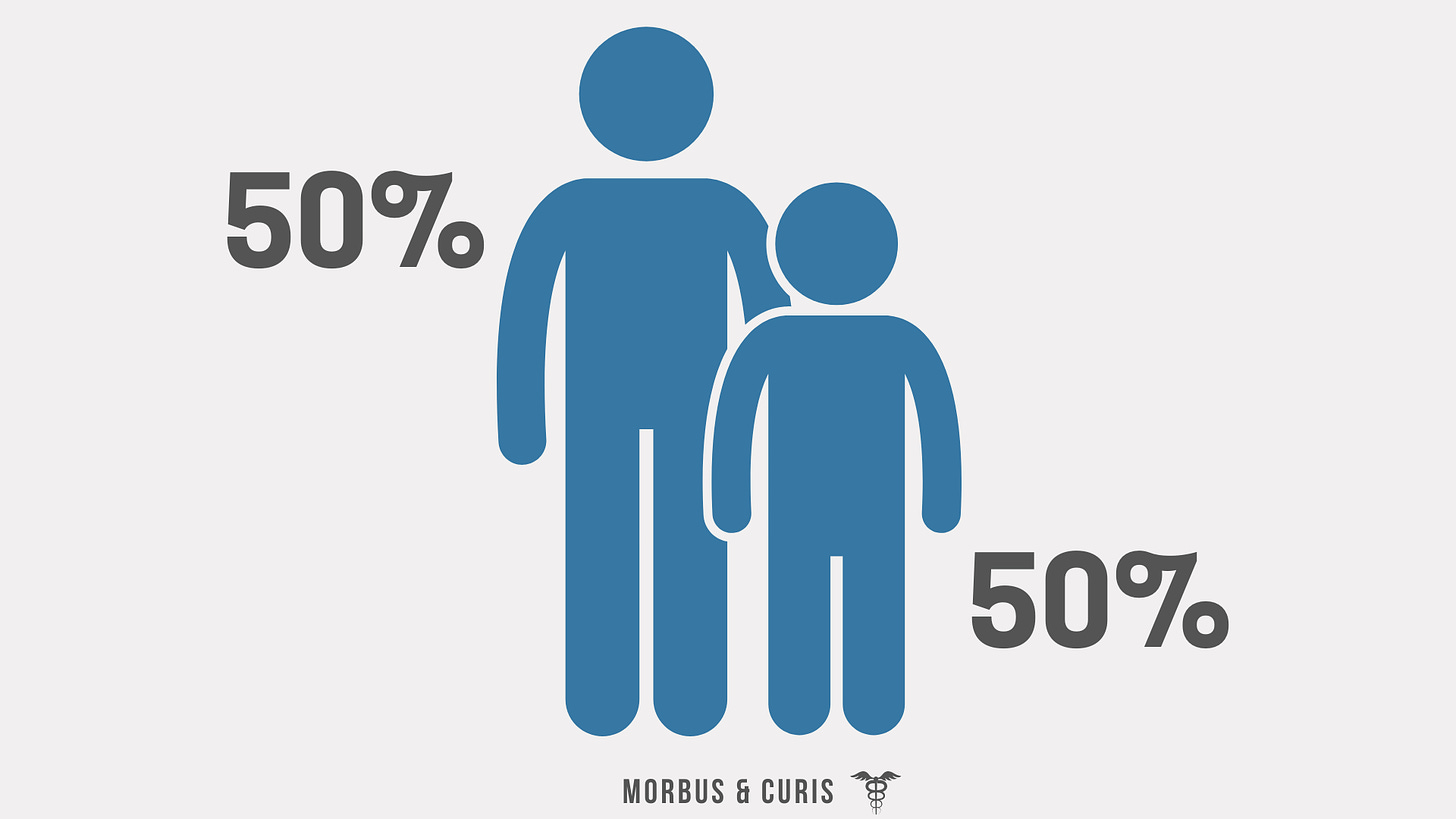
Approximately 50% of those living with a rare disease are children.
What are the causes of rare diseases?
The causes of rare diseases are varied and some are still unknown.
Of the known causes, the majority are genetic and can be linked to a mutation in a single gene. Other causes include environmental factors, allergies or infections.
Examples of rare diseases caused by genetic mutations are:
Cystic Fibrosis
Huntingdon’s Disease
Muscular dystrophy
Certain inherited types of cancer
Example of rare diseases caused by environmental factors include:
Malignant mesothelioma (which is caused by exposure to asbestos)
Buerger's disease (development of the disorder is associated with tobacco use)
Keshan disease (caused by a diet deficient in selenium)
Diagnosis and treatment of rare diseases
Diagnosis
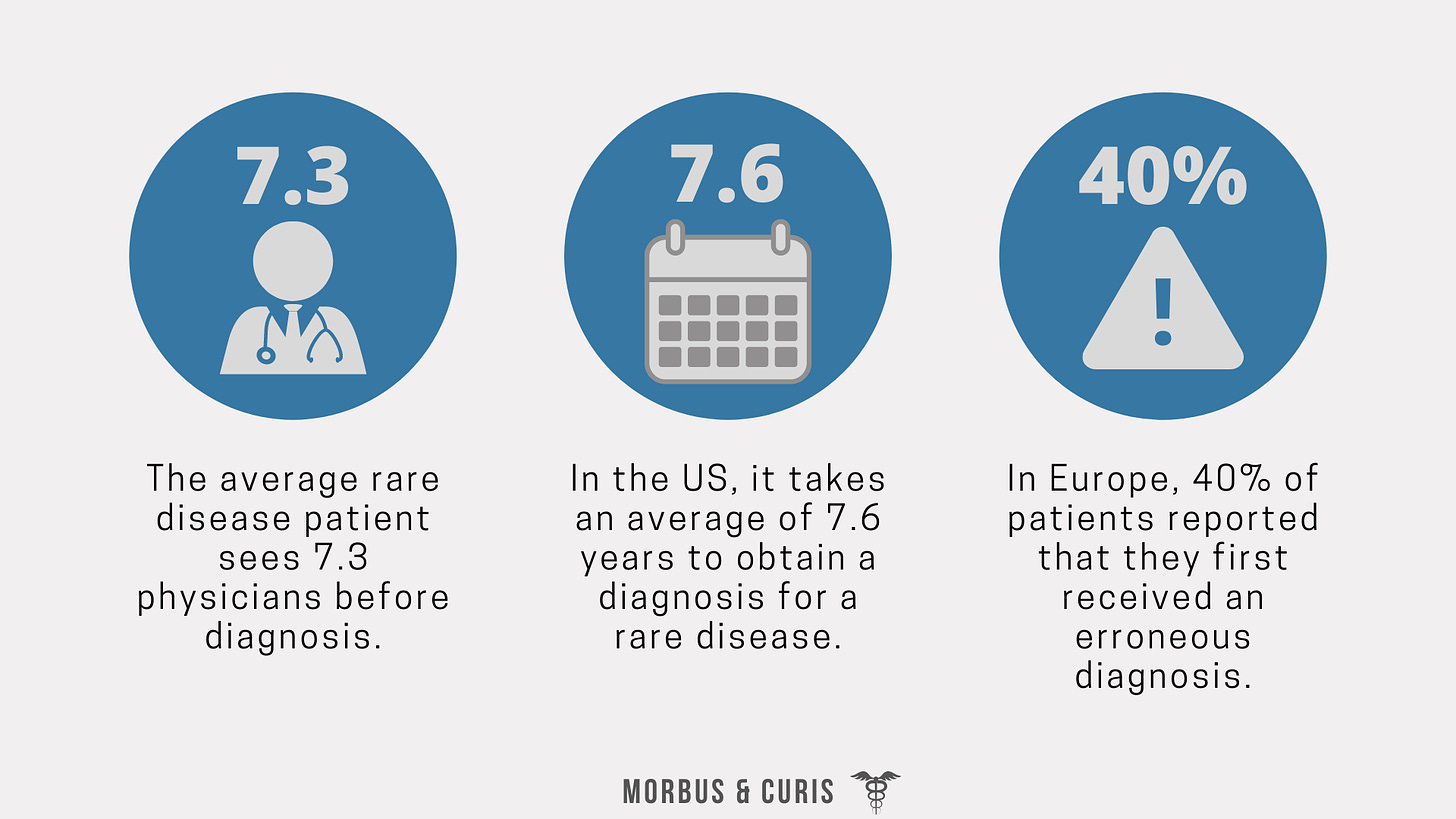
A common issue for people living with rare diseases is delayed or erroneous diagnosis. Studies and surveys have reported:
Reported challenges associated with the diagnosis of rare diseases include:
A lack of rare disease knowledge
A lack of understanding of where to refer patients
Symptoms, severity and course of disease often differing widely between patients
Improving the diagnosis of rare diseases is of high importance to improve patient outcomes and to avoid patients receiving inappropriate medical interventions.
Treatment
Medicines that treat rare diseases are known as orphan medicines.
Today, only ≈5% of rare diseases have licensed treatment options but recent advancements in targeted therapies have led to the development of innovative treatments for several rare diseases and may continue to help in the discovery and development of more new treatments.
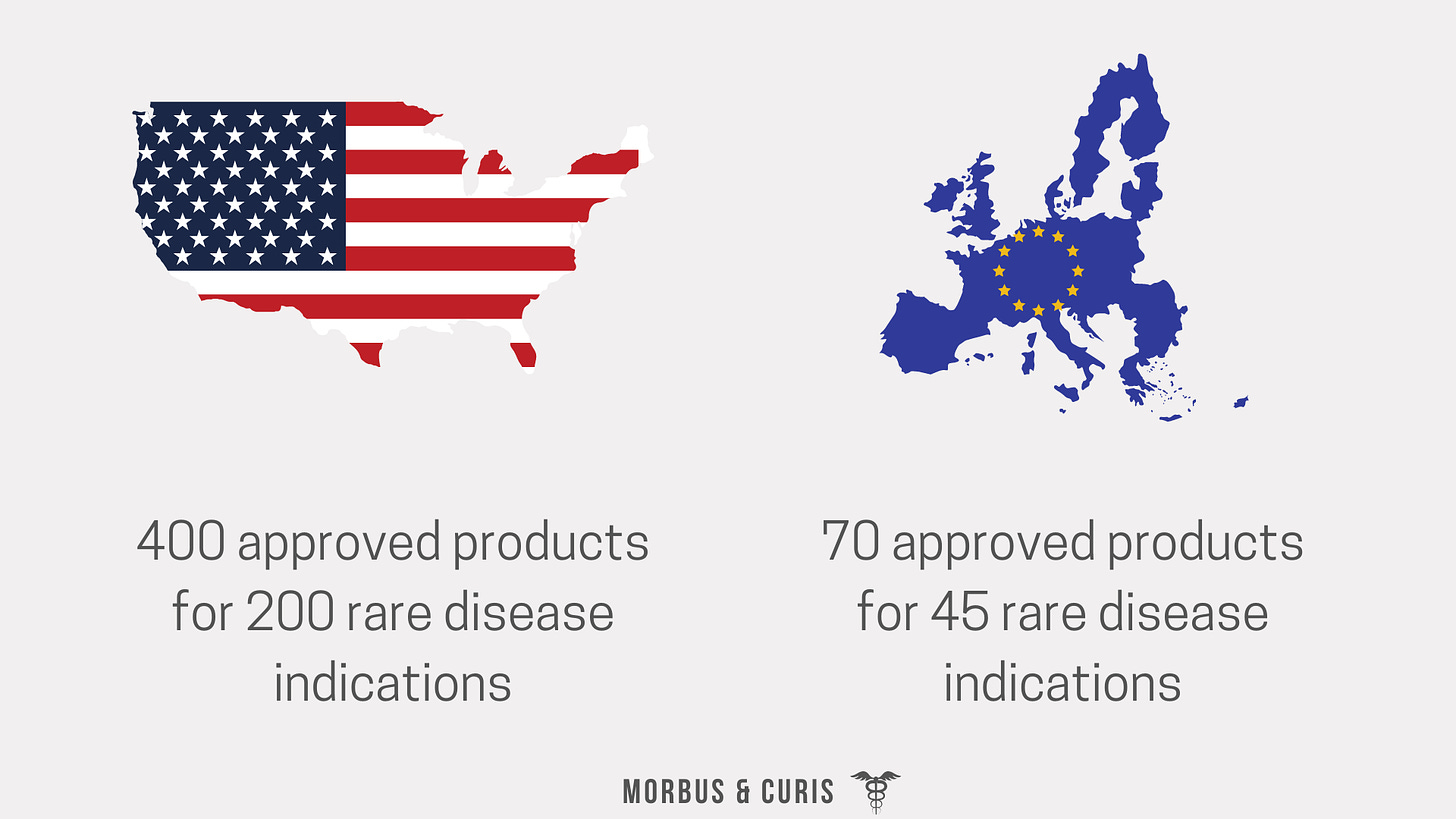
Access to treatments for rare diseases varies across the globe. In the US, there are treatment options for over 200 rare diseases but in Europe, options are more limited with only 70 medicines having been approved for 45 rare diseases.
Rare disease clinical trials and R&D assistance programmes
Some of the key challenges associated with conducting clinical trials for rare diseases include:
Difficulties conducting clinical trials for small, widely dispersed patient populations as well as paediatric populations
Lack of validated biomarkers and surrogate end-points
Limited clinical expertise
Also, under normal market conditions, it would not be profitable to develop a medicine for such a small patient population.
Consequently, to mitigate some of the challenges and risks associated with drug development for rare diseases, various financial incentives and assistance programmes have been established to support the development of orphan medicines.
In the EU, orphan medicine incentives and assistance include:
Protocol assistance: scientific advice regarding the types of studies needed to demonstrate the medicine's quality, benefits and risks.
Access to the centralised authorisation procedure: orphan medicines are assessed centrally, which allows the manufacturer to make a single application for approval in all EU Member States.
Ten years of market exclusivity: orphan medicines are granted ten years of protection from market competition.
Fee reductions: manufacturers pay reduced fees for regulatory activities for orphan medicines.
Read more here.
In the US, the Orphan Drug Act was signed into law in 1983 and has since supported the development of over 340 treatments for rare diseases. The Orphan Drug Act allows manufacturers to receive 7 years of marketing exclusivity in the US and a 50% tax credit for expenditures incurred while orphan drugs were being evaluated for their therapeutic potential.
Sources
If you found this blog post helpful why not consider subscribing or sharing this post.