What is CRISPR technology?
CRISPR-Cas9 technology has been described as precision surgery for DNA. Let's deep dive on how it works, using the recently approved CASGEVY™ as an example.
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), also known as CRISPR-Cas9, is a gene-editing tool. It allows scientists to edit genes in living cells, by finding and fixing gene mutations.
It has been described as precision surgery for DNA since it allows scientists to cut out gene mutations, which can then be fixed or turned off.
How it works
There are two key components to CRISPR-Cas9 technology – a guide RNA (gRNA) and the Cas9 enzyme, and the process is as follows:
First, a gene mutation requiring editing is identified by scientists, and a guide RNA is designed to exactly match the target gene’s DNA sequence.
The gRNA and Cas9 enzyme are inserted into the cell, after which the gRNA binds to the Cas9 enzyme and guides it to the target gene for editing.
The Cas9 enzyme cuts both strands of the DNA at the target location.
What happens next can vary, but all outcomes leverage the cell's DNA repair mechanisms to:
Insert new DNA to correct a gene mutation.
Turn off a gene by removing a section and creating a frameshift mutation.
After the DNA is repaired, the process of editing the gene is complete.
Fun fact: the inventors of CRISPR were awarded the Nobel Prize in 2020.
Are there any approved CRISPR technologies?
Recently the Medicines and Healthcare products Regulatory Agency (MHRA) approved the first gene therapy using CRISPR technology, CASGEVY™. This was not just a UK first but also a world first.
CASGEVY™ was granted conditional marketing authorization (CMA), which will be valid for one year but is renewable annually with ongoing regulatory review of data. CMAs are typically granted to medicines for which comprehensive clinical data is not yet available but early data indicates that the medicine addresses a significant unmet medical need.
What is Casgevy?
CASGEVY™ (exagamglogene autotemcel [exa-cel]) is a gene therapy indicated for the treatment of sickle cell disease and transfusion-dependent β-thalassemia for patients aged 12 and over. Both of these diseases are caused by gene mutations in the haemoglobin beta-chain gene, which affect the ability of red blood cells to carry oxygen around the body.
How does it work?
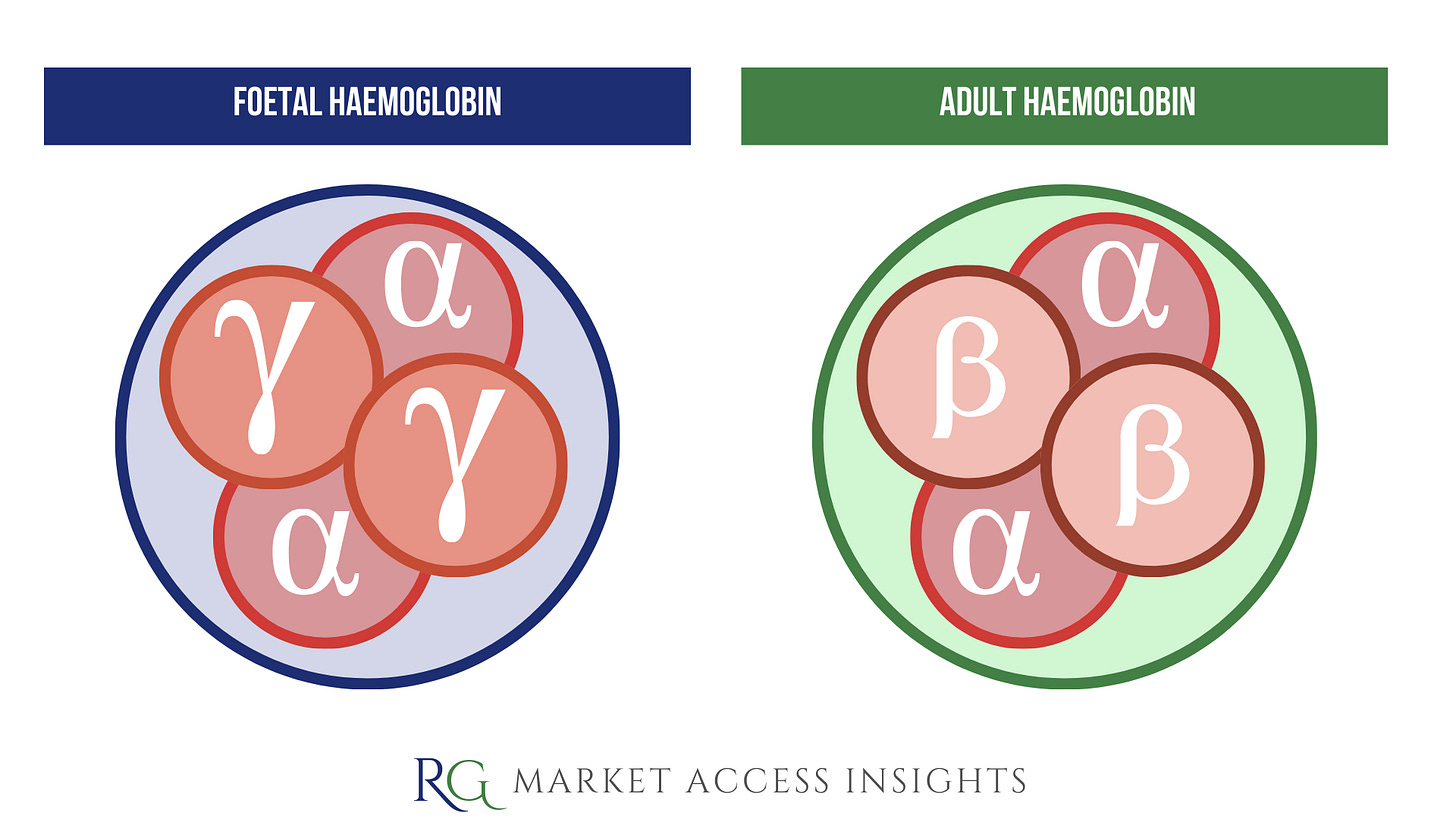
CASGEVY™ works by editing a transcription factor gene in a patient’s bone marrow stem cells so they can switch on the production of foetal haemoglobin (HbF).
Foetal haemoglobin contains two alpha-globin and two gamma-globin protein chains, whereas adult haemoglobin (HbA) contains two alpha-globin and two beta-globin protein chains.
The production of foetal haemoglobin is switched off after birth, as the body switches to producing the adult form of haemoglobin. Patients with SCD or TDT produce defective or insufficient adult haemoglobin.
It’s anticipated that CASGEVY™, by boosting levels of foetal haemoglobin, can compensate for the defective adult haemoglobin thereby alleviating painful sickle cell crises or the need for blood transfusion for SCD and TDT patients respectively.
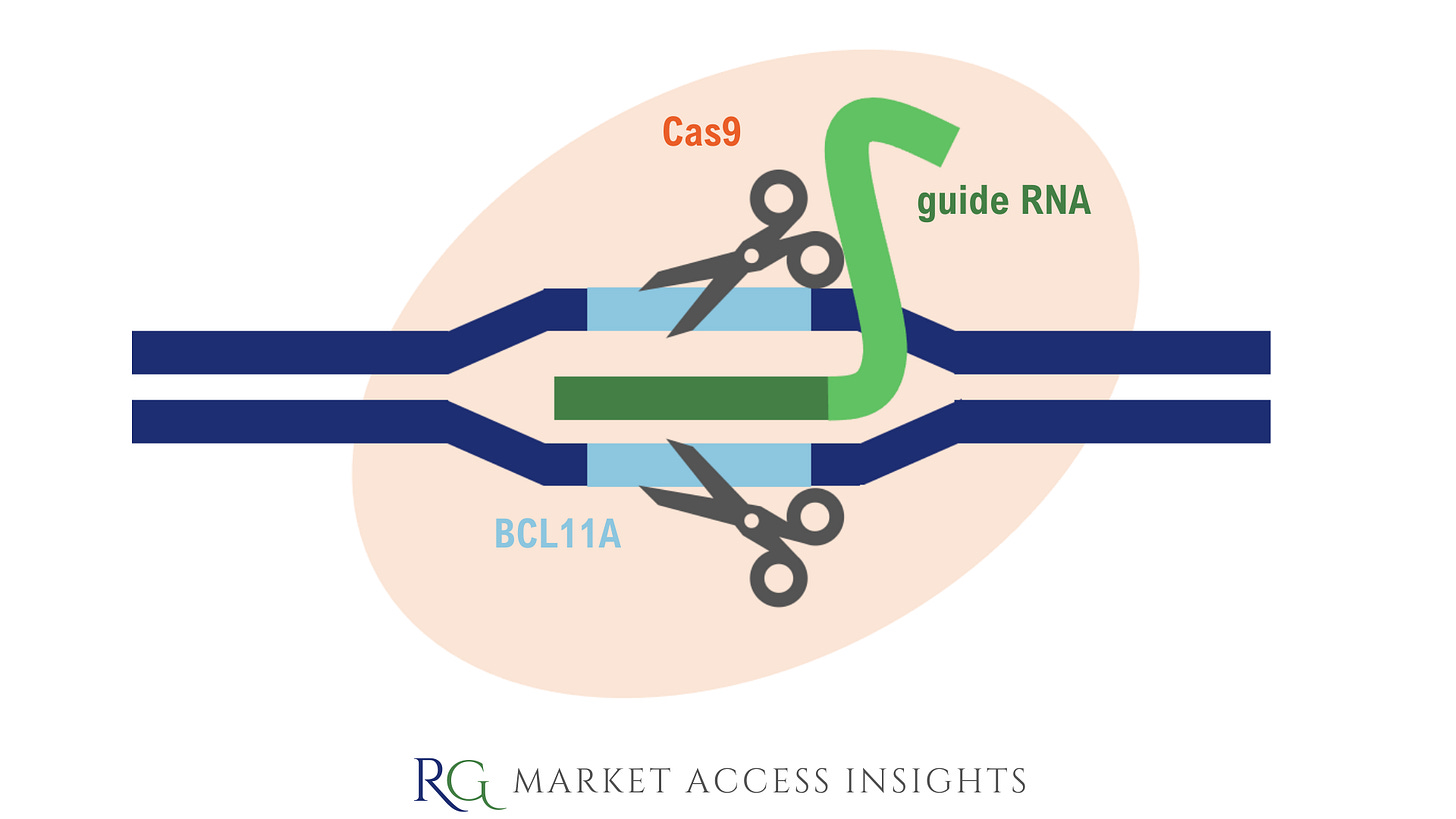
During treatment with CASGEVY™, a patient’s hematopoietic stem and progenitor cells are edited ex vivo by CRISPR/Cas9 at the erythroid-specific enhancer region of the BCL11A gene.
The BCL11A gene acts as a repressor of foetal haemoglobin genes; it binds to DNA near the HbF genes and turns them off so adult haemoglobin can take over after birth.
CASGEVY™ targets BCL11A and lifts its repression on HbF production, allowing the production of foetal haemoglobin that doesn’t carry the defects found in adult haemoglobin in people with SCD or TDT.
Administration
A patient’s blood-producing stem cells are extracted from their bone marrow and then edited in a laboratory using CRISPR–Cas9.
Once the gene editing process on the BCL11A gene is complete, the patient undergoes conditioning treatment so the modified stem cells may be infused back into them.
If treatment is successful, the modified stem cells will take up residence in the patient’s bone marrow and produce a stable form of foetal haemoglobin. This process takes time and patients may need to spend a month in hospital following the administration of the modified stem cells.
Clinical Trial Results
In both global clinical trials of CASGEVY™ (CLIMB-121 for SCD and CLIMB-111 for TDT), the majority of patients included in the interim primary efficacy analysis met their respective primary outcomes of becoming free from severe VOCs or transfusion-independent for at least 12 consecutive months.
Sickle-cell disease (CLIMB-121)
45 patients have currently received CASGEVY™.
29/45 (64%) patients have been in the trial long enough to be included in the primary efficacy interim analysis.
28/29 (97%) of those patients were free of severe pain crises for at least 12 months after treatment. (Source.)
Transfusion-dependent β-thalassemia (CLIMB-111)
54 patients have currently received CASGEVY™.
42/54 (78%) patients have been in the trial long enough to be included in the primary efficacy interim analysis.
39/42 (93%) did not need a red blood cell transfusion for at least 12 months after treatment. The remaining 3 had >70% reduction in the need for red cell transfusions. (Source.)
The safety profile of 97 SCD and TDT patients treated to date with CASGEVY™ in these clinical trials is generally consistent with myeloablative conditioning with busulfan and hematopoietic stem cell transplant. (Source.)
Both clinical trials are ongoing and further results will be made available in due course. Additionally, the safety and effectiveness of CASGEVY™ will be closely monitored through real-world safety data and post-authorisation safety studies conducted by Vertex.
Both sickle cell disease and β-thalassemia are painful, life-long conditions that in some cases can be fatal. To date, a bone marrow transplant – which must come from a closely matched donor and carries a risk of rejection – has been the only permanent treatment option. of-its-kind gene-editing treatment called Casgevy, which in trials has been found to restore healthy haemoglobin production in the majority of participants with sickle-cell disease and transfusion-dependent β -thalassaemia, relieving the symptoms of disease.
Julian Beach, Interim Executive Director of Healthcare Quality and Access at the MHRA
What’s next?
CASGEVY™ is currently being assessed by NICE via the standard technology assessment (STA) process and NICE’s recommendation is anticipated in April 2024. It’s estimated that there are 2,000 patients who meet the eligibility criteria for CASGEVY™ in the UK.
Although no list price is yet available for the UK, the general trend for gene therapies is that either a patient access scheme or commercial arrangement is needed to improve cost-effectiveness and enable patients to gain access to these high-cost treatments.
Elsewhere, CASGEVY™ is under review by the European Medicines Agency (EMA) and the Food and Drug Administration (FDA).
CRISPR Concerns
While the early medical results may be promising there are still some scientific, ethical and social concerns regarding the use of CRISPR.
One concern relates to off-target effects. These occur when a CRISPR tool makes unintended cuts in other genes that are similar to the target gene and could cause unintended gene mutations. The risk of this occurring will reduce as the specificity of guide RNAs is improved but it’s not yet perfect.
Additionally, there are ethical and safety concerns about the regulation, accessibility and use of gene editing. For example, there are concerns gene editing could be used to enhance traits instead of treat disease, which could deepen inequalities or have unforeseen consequences not yet identified through scientific research.
As the gene therapy landscape grows and evolves, continued monitoring and debate will be needed to ensure proper oversight and regulation.
If you found this blog post helpful, consider sharing it or subscribing :)